
Gut Health and its importance for optimal Immunity
INTRODUCTION
What a rollercoaster ride these past two ‘Covid’ focused years have been for all concerned. You will no doubt be inundated with patients world over needing your help more than ever. Your patients will likely be presenting with neurological, cardiometabolic, endocrine, digestive and immunological issues, ranging from one symptom to many symptoms across all systems. As we know, these systems are interrelated, and none work effectively without the other. When it comes to the immune system, gastrointestinal (gut) health is paramount.
When this gut-immune gateway is not working as it should, an array of other problems may start occurring – joints may ache, blood glucose levels may not be properly regulated, moods can be unstable, hormones may become unbalanced and skin rashes can develop seemingly out of nowhere.
If your patient presents with reoccurring food intolerances, candida, viruses, parasites, bacteria such as E. Coli, H. pylori and other gram-negative bacteria, despite the usual BICOM bioresonance systematic treatment (basic therapy, blockage programmes, detoxification programmes, inversion of the stressor followed by stabilisation), we need to question the state of the gut-immune homoeostasis. Rather than putting out the ‘fires’ here, there and everywhere, it is important to correct immune dysfunction within the gut as well as at a cellular level.
As the father of modern medicine, Hippocrates BC, stated ‘all disease begins in the gut’.
Understanding gut immunity
The gut should function with the continuous challenge of responding to pathogens whilst remaining relatively unresponsive to commensal microbiota, food proteins and other antigens. Yet if inflammation is at play, this ability can be significantly compromised.
A major contributor is the close interrelationship among five key elements of the gut ecology – the intestinal epithelium and its overlying mucous membranes, its underly- ing immune cells, the gut microbiota, the capillary bed and the neural networks that enable communication from the gut to other body systems.
Every day we seem to be learning more and more about the relationship between the gut microbiome and immune health. In fact, it is the microbiota within the microbiome that shapes the innate and adaptive immune response as well as impacting systemic immune responses to achieve homeostasis.
Digestive health is tied to immune function, it is estimated that 70% of our entire immune system resides just below the layer of cells lining the gut. This intricate immune system consists of gut-associated lymphoid tissue (GALT) and is part of the larger mucosal-associated lymphoid tissue (MALT) which includes immune cells in the respiratory, digestive, and genitourinary systems. These GALT areas of tissue, referred to as Peyer’s Patches, are found prominently throughout the intestinal tract and are critical in protecting us from pathogenic or opportunistic microbes (bacteria, fungi, protozoa, and viruses) and toxic material that may be in our food and water supply.
Innate Immunity and the microbiota
The cells involved in the innate immune system have co-evolved with the microbiota. These include:
Antigen-presenting cells (APCs) – A group of immune cells that present antigens to T cells and lymphocytes. The gut microbiota regulates the development of APCs. These cells connect innate and adaptive immunity.
Neutrophils – The microbiota is recruited to regulate neutrophils, which make up 40-60% of white blood cells.
Natural killer (NK) cells – Secrete cytokines and act on other cells such as macro- phages to mount an immune response. There is much crosstalk between NK cells and the microbiota as they work together to promote health and ward off disease.
Mast cells – Cells that contain histamine and heparin, part of the immune and neuroimmune systems. These act at the intersections between intestinal mucosa, microbiota and the nervous system.
Intestinal/gut epithelium – A single cell layer that plays an important role in the immune system from conception, forming a physical and chemical barrier to protect intestinal mucosa, while working symbiotically with the microbiota to quickly remove damaged cells.
The challenge of the gut epithelium is to:
Provide a protective barrier
Counter the natural tendency towards a proinflammatory state (which can lead to systemic issues).
Maintain control via the tight junctions
Prevent intrusion of unwanted pathogens and toxins
Allow easy passage of nutrients

Adaptive immunity and the microbiota
The gut microbiota plays an important role in the development of white blood cells, such as T cells. The two key types of cells involved in the adaptive immune system are:
B-cells mature in the bone marrow and produce antibodies in response to the antigens. B-cells are involved in humoral response. As soon as B-cells come across the antigens, they produce plasma cells and memory B-cells. Most are immunoglobulin A (IgA) secreting plasma cells, a type of antibody that plays a critical role in the immune function of mucous membranes. The microbiota is a major driver of IgA production.
T-cells originate in the bone marrow and mature in the thymus. These can be further divided into T-helper cells and T-cytotoxic cells. They are responsible for removing the pathogens from the body. As soon as the foreign antigen enters the cells, T-cells trigger the B-cells to develop plasma cells and activates T-killer cells that kill the cells affected by the invaders.
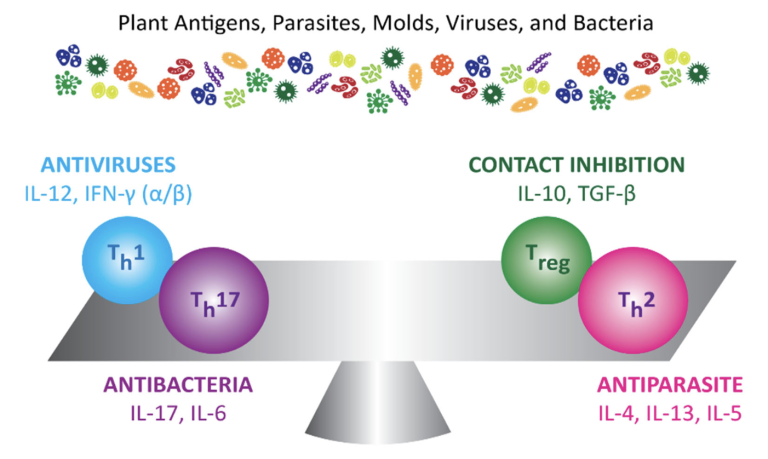
TH1 and TH2 T cells
TH1 is the pro-inflammatory side of the immune system — it responds immediately to an invader entering the body. TH2, on the other hand, is the anti-inflammatory side of the immune system. After a delayed response, TH2 produces antibodies to combat an invader. These antibodies tag the invader so that if it shows up again, the immune system can respond more quickly. In a healthy person, these two systems work in balance. In a person suffering from an autoimmune disease, however, one of these systems may become overly dominant. This imbalance between TH1 and TH2 underlies autoimmune conditions.
The new immune player: TH17
TH17 is another important player in the immune system. When activated properly, TH17 plays an important role in immune defence. When it over-activates, however, TH17 becomes a factor in autoimmune disease and chronic inflammatory disease.
From a scientific standpoint, TH17 is activated by IL 6, which increases when blood sugar levels drop and when stress responses increase — such as in times of psychological and emotional stress. When TH17 is activated, it will then turn up IL 17, which activates Nuclear Factor Kappa Beta (NF-kB). NF-kB is a pro-inflammatory gene transcription factor that is also activated by poor tight junction control, dysbiosis, toxins, food sensitivities, stress and pathogens. All of this can play a role in triggering immune dysfunction and autoimmune conditions.
The most important T-cell: Regulatory T-cells (TREG)
One of the most important things to re-balance the immune system is to support regulatory T-cells via the gut microbiome (also known as TH3 cells). These cells keep all facets of the immune system in check by regulating the activity of TH1, TH2, and TH17. When the T-cells don’t function properly, the immune system can tip out of balance and promote inflammation and autoimmunity.
When the immune system becomes dysregulated due to T-cell problems, autoimmunity may develop – a loss of self-tolerance at a cellular and tissue level. Increasing regulatory T-cells is key to restoring self-tolerance and restoring balance to the immune system.
When applying bioresonance to managing immune dysfunction and inflammation, we focus on identifying why the immune system is imbalanced and then work to restore that balance including helping to hinder uncontrolled inflammation and re-establish a healthy gut microbiome with healthy eating and nutrigenomics.
Germ vs. terrain
The fight against infectious diseases advanced dramatically with the establishment of the germ theory in the 19th century. This theory is based on the concept that germs are the enemy and therefore must be directly attacked or avoided. This theory is be- coming increasingly challenged against the “terrain theory”.
We now know a focus solely on fighting germs is insufficient. The ‘terrain theory’ pro- poses that disease is less likely to occur when the host is healthy. That is, unfavourable conditions encourage disease e.g. poor diet and lifestyle, smoking, toxins, constant stress, cold air etc.
In other words, the dynamics of the host immune system plays an important role in defending against infectious diseases.
The “terrain theory” is more consistent with the concepts of Bioregulatory medicine. That is, unfavourable conditions encourage disease, so the focus is on improving over- all health.
How food impacts our DNA
Modern nutrition science has shown us that food provides us with so much more than proteins, fats, carbohydrates, vitamins and minerals. Plant foods in particular, are a rich source of thousands of compounds called phytochemicals (phyto means plant) and it is these that contribute to our health and out gut microbes in so many important ways.
It has been discovered that these phytochemicals send powerful messages to the DNA in our cells, activating the defence mechanisms that protect ourselves from harm. On the other hand, unhealthy processed foods send messages to our cells which increase inflammation.
Detoxification of unwanted chemical substances is an essential function of all cells. In fact, optimal detoxification is one of the most important ways in which cells can protect themselves against developing cancer.
Such naturally occurring compounds capable of switching on hundreds of cell protective genes at the same time is surely an opportunity to acknowledge the marvels of nature!
Introducing Nrf2
Nrf2, short for ‘nuclear factor erythroid 2-related factor 2’, is a protein that is found in our cells. It is a type of protein called a ‘transcription factor’, which means that it is in- volved in gene expression—activating and deactivating parts of a genetic sequence.
Nrf2 has been found to be an important part of your body’s system of regulating metabolism, inflammation, and immune responses.
What does Nrf2 do?
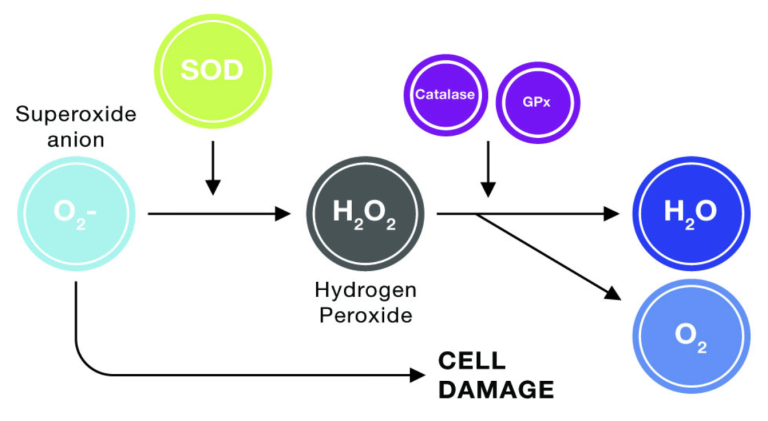
Nrf2 provides a powerful solution to overcome free radicals. It’s known as the “master regulator” of our body’s antioxidant response.
When Nrf2 is activated, it enters the nucleus and turns on several hundred protective genes. This, in turn, initiates the production of several of your body’s own powerful enzymes that fight free radicals. These enzymes include catalase, glutathione, and super- oxide dismutase (SOD).
These enzymes are much more effective than supplemental antioxidants at neutralising free radicals—they can neutralise over 1 million free radicals a second. This is compared to one antioxidant molecule (from secondary antioxidants) which can only neutralise one free radical.
You actually don’t need to consume Nrf2—it’s already right there in your cells. It comes preinstalled.
The problem is that it’s not activated. It’s a bit like an engine: it just sits dormant in your cells not doing anything until it’s turned on. Utilising nutrigenomics and bioreso- nance, it is possible to ‘switch’ on Nrf2 so that it can direct your DNA to turn on hun- dreds of protective target genes.
This pathway, fundamental to all animal cells, is a dynamic response induced by oxida- tive or chemical stress on the cell. It is a switch-on, switch-off mechanism, and most of the known priming agents that facilitate its action are phytochemicals.
Key phytochemicals with Nrf2 priming action include sulforaphane from broccoli sprouts (part of the cruciferous vegetable family), curcumin from turmeric, resveratrol, canosol from rosemary, Gingko extract, polyphenols from green tea, and the sulfur compounds in garlic. 1
Even dark chocolate and red wine are rich in these phytochemicals!
Health and immune benefits of NRF2
Obviously, harnessing a mechanism as powerful as the Nrf2 pathway can have pro- found effects on health maintenance and disease management. Current research has identified many important benefits to priming the pathway in the quest to maintain health and prevent disease 2. These include
Antioxidant activity (and anti-ageing)
Detoxification of toxins, heavy metals etc
Anti‐inflammatory – benefits in diseases involving oxidative damage, autoimmune reactions and inflammation
Anti‐microbial effect by activating beta-defensin (produced by epithelial cells lining various organs)
Facilitating protection against radiation and EMF stress
Reducing cancer incidence and recurrence
Reducing neurodegeneration (and brain fog)
Improving cardio metabolic disorders
Maintenance of mitochondrial homeostasis (relevant for oxidative and inflammatory stress)
The pathway also up regulates glutathione levels, in itself an important cellular antioxidant and protective agent.
The discovery of this pathway is revolutionary and is a game-changer concept, especially for cytoprotection and detoxification.
1 https://pubmed.ncbi.nlm.nih.gov/26881038/
2 Tu.W, Wang H, Li S, Liu Q, Sha H. The anti-inflammatory and antioxidant mechanisms of the Keap 1/Nrf2/ACRE signaling in chronic diseases. Aging Dis. 2019; 10(3): 637-651 http://www.agingand- disease.org/EN/10.14336/AD.2018.0513
Theopposite of NRF2: NF-kB
As mentioned, NF-kB (NF-kappaB) is a protein complex that controls transcription of DNA, cytokine production and cell survival.
Just as Nrf2 is a transcription factor in the cell’s cytoplasm, so too is NF-kB. Where Nrf2 effect is to activate protective genes of the cells defence system and reduce/inhibit in- flammation, NF-kB activates the genes which promote inflammation. Unfortunately, in patients with chronic underlying inflammation, this ‘switch’ gets stuck in the ‘on’ posi- tion.
Chronic inflammation is a situation which underpins many diseases
The good news is that we have answers to both problems. We do know to a large ex- tent what the factors are that trigger the inflammatory process. Mother Nature has provided us with phytonutrients (as previously mentioned) which can inhibit or switch off uncontrolled NF-kB. Knowing how to regulate the process in both ways helps us to take control of diseases we may have thought we had to live with.
Having the ability to turn down NF-kB to reduce the tendency for inflammation and turn up Nrf2 to help cells to protect themselves provides us with a powerful weapon against disease, whatever that disease may be named.
Covid‐19 and the gut
Wading through these years of unchartered territory with Covid-19, as practitioners, we find ourselves needing to support our patients gut and immune health even more.
Interestingly, studies examining the effects of SARS-CoV-2 infection in the gut, have identified differences between the gut microbiome of patients with Covid-19 against persons without Covid-19.
Generally, SARS-CoV-2 positive individuals showed depleted Bifidobacterium and Rose- buria, both of which exert anti-inflammatory properties. (Roseburia is a member of the beneficial Firmicutes phylum of butyrate-producing, Gram-positive anaerobic bacteria that inhabit the human colon.)3
There is also evidence that the spike protein associated with SARS-CoV-2 and the Covid-19 vaccines can impact the epithelium and endothelium. It appears the spike protein target the ACE2 receptors, which are highly expressed on the luminal surface of intestinal epithelial cells. 4
3 https://www.frontiersin.org/articles/10.3389/fcimb.2022.848650/full
4 https://www.gastrojournal.org/article/S0016-5085(20)35327-0/fulltext?referrer=https%3A%2F%2Feuropepmc.org%2FThis may partially explain why some patients who are symptom-free following COVID infection have been found to have the spike protein residue persist in the faecal matter for an extended period of time5. A new pathogen exposure and/or reactivation of stealth pathogens (e.g. Herpes viruses, mycoplasma, Streptococci etc) may result in a return of COVID symptoms in such persons if the virus and or corresponding vaccine has not been completely treated and cleared (energetically speaking).
Large intestine and lung meridians
Emerging evidence also highlights an important crosstalk between the gut microbiome and the lung. Alterations in the gut microbiome may impact on lung immunity in the context of pulmonary diseases6. This makes sense from a TCM perspective when we consider the lung and large intestine meridians, both meridians assigned to the METAL element.
Gut Brain Connection: The relationship to emotions
An anxious mind can create stress in the body causing upset or disharmony in the gut, contributing to immune dysfunction and inflammation. Similarly, problems in the gut can cause an imbalance in the mind. These two entities are continually in communication with each other.
The gut is so highly innervated that it has its own dedicated nervous system, the enteric nervous system (ENS). The parasympathetic nervous system (PSNS) and ENS pro- vide a feedback system to each other via the vagus nerve (a cranial nerve that innervates the heart, lungs, and almost the entire gut). They are also connected through the limbic system (hypothalamus and amygdala), the area of the brain involved in memory and emotional response.
The vagus nerve conveys messages in both directions. The vagus nerve is the major neural connection between gut and brain. Signals are sent via the nerve into the brain and the brain transmits signals to the peripheral body and gut. Gut instincts and visceral sensations are transported up to your brain via the vagus nerve. It is the translator from the gut to the brain.
Under stress, the vagus nerve impacts the parasympathetic nervous system which manages our ‘rest and digest’ response. Stimulation of the vagus nerve increases the vagal ‘tone’, meaning your body can relax faster after stress reducing cortisol levels. Swimming, singing humming and gargling can increase vagal tone.
Additionally, many neurotransmitters directly act via the gut-brain axis, such as dopa- mine, adrenaline and noradrenaline. Large amounts of serotonin are also produced in the gut.
5 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7736111/ 6 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7052046/
As practitioners, it is important to address not just the physical body when identifying the cause of immune dysfunction, but also to look at the role of energetic blockages and trapped emotions, often initiating the patient’s physical symptoms in the first place. We can consider chakra therapy, meridian balancing, and shock programmes as examples.
The bottom Line
As we learn more about the complex interplay between the immune system and gut, we can take more decisive action to optimise the health and wellbeing of our patients. Our job is becoming increasingly directed toward cellular health, antioxidant and boosting microbial health to ensure that all systems in the body function effectively.
